Abstract
Introduction: Umbralisib (UMB) is a next generation, once daily, PI3Kδ/CK1ε inhibitor, active in patients with relapsed or refractory (rel/ref) hematologic malignancies that, in long-term follow-up, has demonstrated a uniquely differentiated safety profile from prior PI3Kδ inhibitors (Davids, 2018). Ublituximab (UTX) is a novel glycoengineered mAb targeting a unique epitope on the CD20 antigen. Bendamustine (Benda) is an active chemotherapy agent in pts with lymphoma. The combination of UMB + UTX (U2) is tolerable and active in patients with rel/ref hematologic malignancies and registration directed trials for patients with CLL & NHL are ongoing. This Phase 1 trial evaluates the safety and efficacy of U2 + Benda in patients with advanced diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL).
Methods: Eligible patients had rel/ref DLBCL or FL with an ECOG PS ≤ 2 w/o limit to number of prior therapies. ANC of ≥ 750 and Platelets ≥ 50,000 were required; no growth factor support was permitted in Cycle 1 (cohort escalation group only). Patients refractory to prior PI3Kδ, Benda, or anti-CD20's were eligible. UTX was dosed on Days 1, 8, 15 of Cycle 1, Day 1 of Cycle 2-6, followed by Cycle 9 & 12. UMB was started at 800 mg QD with a -1 dose reduction cohort at 600 mg if not tolerated in ≥ 2/6 patients. Benda was dosed at 90 mg/m2 on Days 1 & 2 of Cycles 1-6 only. Primary endpoints included safety and efficacy (Cheson 2007).
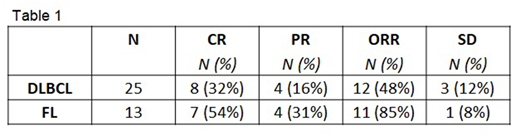
Results: Thirty-nine patients were evaluable for safety: 26 DLBCL and 13 FL. Med age 67 yo (range 31-81); 23 M/16 F; median prior treatment regimens = 2 (range 1-6); 22 pts (56%) were refractory to prior treatment and 6 patients had progressed post-transplant; ECOG PS 0/1/2 (12/25/2). Initially 2/4 patients at 800 mg UMB experienced AE's in Cycle 1 that led to treatment interruption (rash, neutropenia) thus the 600 mg dose of TGR-1202 was explored. No additional Cycle 1 treatment delays were reported at the 600 mg dose level, which was later expanded and the 800 mg UMB dose was evaluated with the use of growth factor support in cycle 1 permitted. The most common AE's regardless of causality included diarrhea (54%; G3/4 15%), nausea (49%; G3/4 5%), vomiting (38%; G3/4 0%), neutropenia (33%; G3/4 33%) and pyrexia (31%; G3/4 0%). Thirty-eight patients (25 DLBCL/13 FL) were evaluable for efficacy (1 DLBCL patient came off study for G4 neutropenia prior to first assessment). ORR in the respective groups is shown in Table 1. The median time to response was 8 weeks. The median DOR was 9.6 months (95% CI: 2.5-NR) for patients with DLBCL, and was not reached (95% CI: 8.0-NR) for patients with FL, at a median duration of follow-up for responders of 11.5 months (range 2.9 - 30+ mos).
Conclusions: The combination of U2 + bendamustine has exhibited manageable toxicity with significant activity in advanced DLBCL and FL patients, including an encouraging CR rate in advanced patients. Based upon the early activity of the triplet, a registration directed study is underway for patients with rel/ref DLBCL (UNITY-NHL).
Lunning:Gilead: Consultancy; Astra-Zeneca: Consultancy; Genentech: Consultancy; Spectrum: Consultancy; TG Therapeutics: Consultancy; Bayer: Consultancy; Celgene: Consultancy; AbbVie: Consultancy; Genzyme: Consultancy; Kite: Consultancy; Juno: Consultancy; Genentech: Consultancy; Portola: Consultancy; Janssen: Consultancy; Seattle Genetics: Consultancy; Verastem: Consultancy. Siddiqi:Juno Therapeutics: Other: Steering committee. Flowers:Abbvie: Research Funding; TG Therapeutics: Research Funding; Gilead: Research Funding; Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; Genentech/Roche: Research Funding; Genentech/Roche: Consultancy; Pharmacyclics: Research Funding; V Foundation: Research Funding; Abbvie: Consultancy, Research Funding; Bayer: Consultancy; Karyopharm: Consultancy; Burroughs Wellcome Fund: Research Funding; Celgene: Research Funding; BeiGene: Research Funding; Gilead: Consultancy; Millennium/Takeda: Research Funding; OptumRx: Consultancy; Pharmacyclics/ Janssen: Consultancy; Spectrum: Consultancy; Janssen Pharmaceutical: Research Funding; Denovo Biopharma: Consultancy; Acerta: Research Funding. Cohen:Millennium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Infinity Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Infinity Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Janssen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioInvent: Consultancy; Takeda: Research Funding; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BioInvent: Consultancy. Blumel:TG Therapeutics, Inc.: Consultancy. Cutter:TG Therapeutics, Inc.: Consultancy. Pauli:TG Therapeutics, Inc.: Consultancy. Sportelli:TG Therapeutics: Employment, Equity Ownership. Miskin:TG Therapeutics: Employment, Equity Ownership. Weiss:TG Therapeutics: Employment, Equity Ownership. Vose:Kite Pharma: Research Funding; Legend Pharmaceuticals: Honoraria; Roche: Honoraria; Incyte Corp.: Research Funding; Bristol Myers Squibb: Research Funding; Novartis: Honoraria, Research Funding; Abbvie: Honoraria; Seattle Genetics, Inc.: Research Funding; Merck Sharp & Dohme Corp.: Research Funding; Acerta Pharma: Research Funding; Epizyme: Honoraria; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal